Scientists Develop "Non-Permanent" Gene Therapy for Pain Treatment

Complete the form below to unlock access to ALL audio articles.
The opioid crisis
Pain is ironic. It is a universal experience that all we endure at some point in our lives, but it is also highly subjective; your experience of pain is unlikely to be identical to that of another person.
This subjectivity, among other factors, makes pain quite a challenging topic to research and understand at the physiological level. Subsequently, progress in our ability to treat pain pharmaceutically – particularly chronic pain – has been slow in recent decades.
According to the National Center for Health Statistics, chronic pain and high-impact chronic pain are amongst the most common reasons why adults seek medical care. Currently, treatment for severe pain often consists of mainly opioids, which can be addictive.
In 2017, the U.S. Department of Health and Human Services (HSS) declared widespread opioid use to be a public health emergency, stating that "increased prescription of opioid medications have led to widespread misuse of both prescription and non-prescription opioids before it became clear that these medications could indeed be highly addictive". In 2019, 1.6 million people had an opioid use disorder in the previous year, and 10.1 million people had misused prescription opioids.
A safe and efficacious alternative to opioids is necessary to provide pain sufferers with relief without the risk of addiction. "Gene therapies represent the new avenue to tackle those hurdles," says Ana Moreno, a bioengineering alumna from the UC San Diego Jacobs School of Engineering.
Targeting the NAV1.7 protein for pain research
Moreno is the first author of a new study published in Science Translational Medicine in which researchers have developed a novel gene therapy using CRISPR-based gene editing to achieve pain relief in mice.1
The concept was born when Moreno – who is now the co-founder and CEO of Navega Therapeutics – was a PhD student in Professor Prashant Mali's lab at UC San Diego. She identified a research paper discussing genetic mutations in individuals that feel no pain. The mutation inactivates a protein known as NaV1.7, a sodium ion channel encoded by the SCN9A gene, which is involved in the pain signaling pathway. Patients carrying this mutation present with the medical condition congenital analgesia, or congenital insensitivity to pain (CIP), an inability to feel physical pain. As desirable as that sounds, the lack of pain awareness often results in an increased likelihood of accumulating wounds and a reduced lifespan.
NaV1.7 has been explored as a potential drug target for the alleviation of pain through therapeutics such as small-molecule drugs and antibody-based therapy, ultimately to no avail.
"Efforts to develop selective small-molecule inhibitors have, however, been hampered because of the sequence similarity between NaV subtypes. Many small-molecule drugs targeting NaV1.7 have accordingly failed because of side effects caused by lack of targeting specificity or their limited bioavailability by the systemic route," the authors of the new study write.
Could gene therapy be the answer to the opioid crisis?
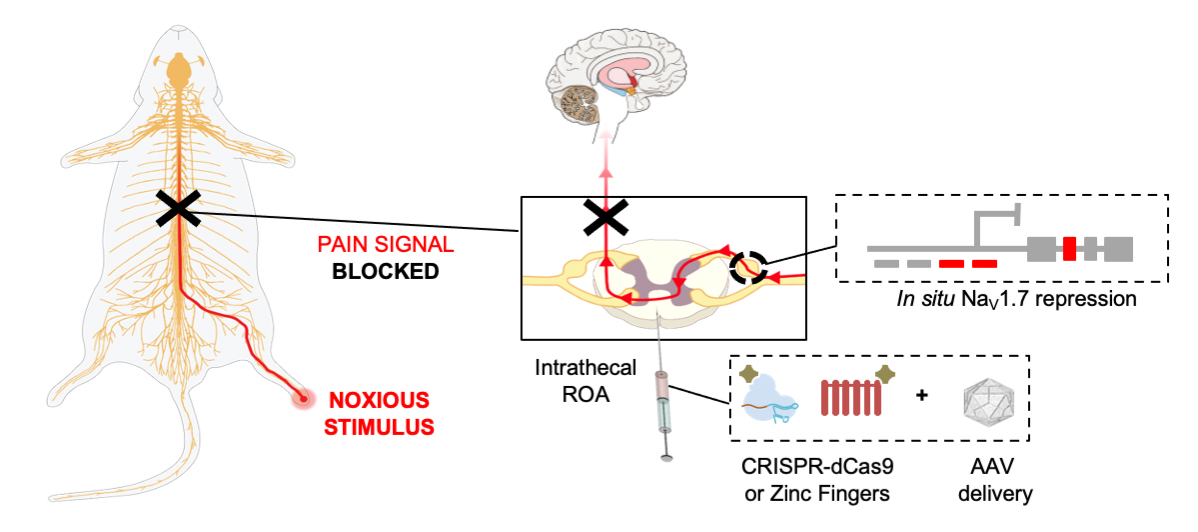
Moreno had been working with a version of the CRISPR-Cas9 technology known as "dead" Cas9, dCas9 or CRISPRi. dCas9 is unable to cut DNA as in traditional CRISPR-Cas9 experiments, but it can bind to the genome via a guide RNA, and "stick" to a specific gene target, ultimately blocking its expression impermanently. What if this so-called epigenetic modulation could be applied to repress the NaV1.7 protein via the SCN9A gene? This would enable the ability to "dampen down" NaV1.7 expression, without shutting it off permanently and risking the adverse outcomes associated with feeling no pain whatsoever.
Moreno and colleagues decided to utilize dead Cas9 to modulate the expression of NaV1.7 to engineer "highly specific, long-lasting and reversible" treatments for pain: "We utilized epigenomic engineering methods that enable transient modulation of NaV1.7 expression. This approach may have lower risk of off-target effects than other approaches," she explains.
Proof of concept for non-permanent gene therapy
First, they tested the "non-permanent" gene therapies' effect in different mouse models of pain compared to control groups. "In our proof of concept, we validated our approach in three preclinical animal models: inflammatory pain, chemotherapy-induced neuropathic pain and BzATP-induced pain," says Moreno.
The dCas9-based gene therapy was administered via spinal injection. The mice demonstrated higher thresholds to pain than the control groups, measured as a slower response to withdrawing their paw from a painful stimulus, and less time spent shaking or licking their paw after the exposure. The effect was maintained after 44 weeks in the mouse model of inflammation, and 15 weeks for the chemotherapy-induced pain model.
Moreno and colleagues chose to validate their findings by utilizing an alternative gene editing tool known as zinc finger protein, which is older and more established than dead Cas9 and serves the same function to repress gene expression. "To validate the results with CRISPR/dCas9 we used zinc finger proteins fused to KRAB repressors. We obtained similar reduction in pain for all the models we tested," says Moreno.
Credit: A.M. Moreno et al. Science Translational Medicine (2021).
In theory, both methods – dCas9 and zinc finger protein-based gene editing – could be further developed to explore their potential as a therapeutic for pain management. "We were excited that both approaches worked," says co-senior study author Mali in a press release. "The beauty about zinc finger proteins is that they are built on the scaffold of a human protein. The CRISPR system is a foreign protein that comes from bacteria, so it could cause an immune response. That's why we explored zinc fingers as well, so we have an option that might be more translatable to the clinic."
A major bottleneck to the introduction of CRISPR-Cas9-based therapies into human medicine is the risk of adverse effects from permanently turning off a gene. When asked if there are any known adverse effects associated with repression of NaV1.7, Moreno says: "Humans with a natural mutation in NaV1.7 have no other developmental or neurological disorder; they just do not feel pain whatsoever. They also present anosmia or a reduced sense of smell. Therefore, if we are specific enough, we do not expect any severe adverse effects in this sense. In addition, a complete temporal block of pain would only occur if we were able to block all neurons. Based on dosage you can potentially regulate how much you tune down the sensation of pain."
Gene therapy: From lab to the clinic
Moreno co-founded Navega Therapeutics alongside Mali to work on translating the gene therapy approach initially developed at UC San Diego. They are working with Professor Tony Yaksh, professor in anesthesiology and pharmacology at UC San Diego and co-senior author of this publication, to do so.
It's still early days for the technology and there are many hurdles a gene therapy must overcome to get close to authorization and approval for human use by regulatory bodies. "We have received some funding from the NIH and private funding. The next goal will be to finalize toxicity and efficacy studies in higher animals closer to humans before starting clinical trials. We also need to determine for how long the therapy works in humans," says Moreno.
If efficacy is confirmed in human trials, scale-up is another barrier that biological therapies face; ultimately requiring highly advanced analytical technologies and processes that are costly. The outcome? An expensive therapy, in most cases.
How will Moreno and colleagues handle these challenges going forward? "We are witnessing an explosion of gene therapy approaches because they are proving to be effective. Being in the forefront of biotechnology implies that you have to break some barriers," she says.
"Tackling chronic conditions like severe pain adds on the issue of large-scale manufacturing. Luckily, improved manufacturing technologies are emerging. This will help with our other challenge: pricing. We think the mix of better manufacturing practices along with the large target population (compared to rare diseases) will allow us to significantly reduce the price and democratize gene therapies."
Ana Moreno was speaking to Molly Campbell, Science Writer for Technology Networks.
Reference:
1. Moreno AM, Alemán F, Catroli M, et al. Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice. Sci. Transl. Med. 2021;13:eaay9056. doi:10.1126/scitranslmed.aay9056.


